Ready For Sale
Secondhand Tecno-Gaz Tecnoheart Plus AED Defibrillator
Price: USD$ 325,00 Approx: 13.975,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside of Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
- Technical Inspection: Technical inspections are performed by our business partner in the seller's country (where available) or by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 21.08.2025
Tecno-Gaz Tecnoheart plus Automated External Defibrillator Analysis Report
Product Description and Content
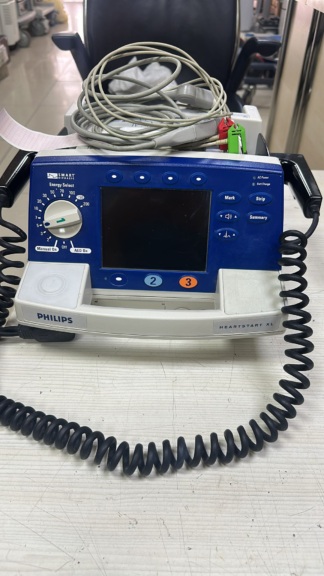
The visuals show a portable Automated External Defibrillator (AED) device and its accessories, designed for use in cases of sudden cardiac arrest. The device is a life-saving equipment that guides the user with audio and visual commands to apply an electroshock. The product package consists of the main unit, defibrillator pads, and a carrying bag. The total number of items in the visual is 3:
- 1 Tecno-Gaz Tecnoheart plus AED device
- 1 Tecno-Gaz defibrillator pad in its sealed packaging
- 1 carrying bag with AED logo
Brand and Model Information
As a result of detailed examinations, the brand and model information of the device and its accessories have been clearly identified as stated below. According to the label information, the device was manufactured by Mediana Co., Ltd. and presented under the Tecno-Gaz brand.
- Device Brand-Model: Tecno-Gaz Tecnoheart plus
- Defibrillator Pad Brand-Model: Tecno-Gaz A1590SGA
General Condition and Physical Assessment
The general condition of the device is very good, and it is observed to be in a near-new condition. There are no significant deformities such as noticeable scratches, cracks, dents, or discoloration on the device. While the device’s carrying bag is also in generally good condition, there are slight white stains on its front surface, which may be due to storage or brief use. The packaging of the defibrillator pads has not been opened and maintains its sterile integrity.
Technical Details and Accessories
The device features characteristics designed for practical use. On its side, there is an SD Card slot for data recording and software updates. Additionally, a speaker grille for voice commands is present on the device’s casing. The main accessories included with the device are:
- Defibrillator Pads: The package contains a pair of pads suitable for adult and pediatric use, with the inscription “Automated External Defibrillator Pads” on it. The pads are connected to the device, ready for use, and their packaging has not been opened.
- Carrying Bag: A black, protective bag with a shoulder strap, featuring “AED” written in large letters on it, and a transparent window to allow the device’s status to be seen externally.
- Battery: As stated on the device’s rear label, it operates with a non-rechargeable LiMnO₂ battery with a capacity of 15V and 4.2Ah. The physical condition of the battery cannot be observed as it is internal.
Label Information
Important technical information is found on the labels located on the device’s rear surface and the pad packaging. This information verifies the device’s identity and features.
- Manufacturing Date: 2022-04-06
- Serial Number (S/N): 456702202125
- REF Code (Device): A15M-G4-0T
- REF Code (Pad): A1590SGA
- UDI Code: (01)18800003456700(11)220406(21)456702202125
- CE Mark: CE 2797
- Protection Class: IP54
Additionally, while the pad packaging states the manufacturer as “CU Medical Systems, Inc.”, the manufacturer on the back of the AED device is indicated as “MEDIANA Co., Ltd.”
Usage and Compatibility
The visuals and buttons on the device’s front panel are designed for ease of use. The device features a mode switch allowing selection based on the patient’s condition: “Adult” (Adult – over 8 years old or above 25 kg) and “Pediatric” (Pediatric – under 8 years old or below 25 kg). This feature enables the same set of pads to be used for both adults and children, saving time in emergencies.
Potential Risk Assessment
Based on visual inspections, no significant wear, rust, cable damage, or broken parts that could pose a risk of malfunction have been observed on the device. The physical condition of the device is very good, and it can be said to appear well-maintained. Battery life and the expiration date of single-use pads are critically important for the functionality of medical devices. Although the expiration date of the pads cannot be clearly read in the visuals, their sealed packaging is a positive aspect.