Ready For Sale
Secondhand Goldway UT3000A Fetal Monitor
Price: USD$ 100,00 Approx: 4.500,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 18.12.2025
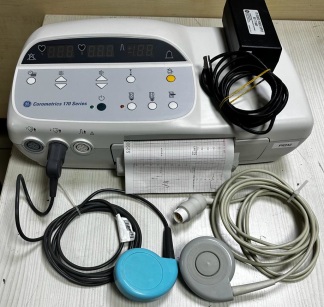
UT3000A Bedside Monitor and Integrated Printer Analysis Report
Device Identification
The examined visuals show a medical device set consisting of two main integrated components. The upper part features a portable, screen-equipped patient monitoring device, while the lower part contains a thermal printer unit designed to be placed under this monitor and compatible with the device. The device is designed in a portable form (portable) with a handle for carrying.
Brand and Model
On the front of the device, the inscription UT3000A is clearly visible on a blue label in the upper left corner of the screen. This code represents the product model. Although the general design of the device and the “UT3000A” model code point to Goldway (or U-Tech in some markets) brand bedside monitors, widely known in the industry, no definite inscription of “Goldway” or the manufacturer’s brand logo is visible in the current angles of the visuals, thus brand information has not been specified for the sake of report objectivity. On the front panel of the bottom unit, the inscription PRINTER appears in italic and uppercase letters.
Areas of Use
This device is used in healthcare institutions (hospitals, polyclinics, emergency services) and ambulances for monitoring patients’ vital functions. Based on the port inputs on the side panel, the device is understood to be capable of the following measurements:
- SpO2: Blood oxygen saturation and pulse tracking.
- NIBP: Non-invasive blood pressure (tension) measurement.
- FT (Fast Temp): Body temperature measurement.
The printer unit at the bottom allows for instant printout of these measurements as a graph or list on paper.
Originality
The placement of labels on the device (“UT3000A”, port labels), the quality of the casing mold, and the standard-compliant design of the port inputs indicate that the product is an original medical device.
Quantity Information
The visuals show a total of one device set, including 1 main monitor unit and 1 printer base unit (docking station). As accessories, only a paper roll installed inside the device and a partially visible black cable are present; external sensors are not shown in the visual.
General Condition and Physical State
The general condition of the device can be classified as “used”. The casing shows a slight yellowing and discoloration (especially on white plastic parts) due to age. However, the external integrity of the device has been preserved. No major cracks or missing parts were detected in the visual.
Physical Deformations and Cleanliness
The most noticeable physical defect of the device is the heavy dust accumulation around the screen and keypad. Specifically, fingerprints and a layer of accumulated dust are present on the screen. Dirt accumulation due to usage can be observed on the orange panel where the buttons are located and around it. No deep scratches or dents were detected on the casing.
Mechanical Components
The mechanical parts of the device are in place:
- The carrying handle on the upper part appears sturdy.
- The 4 push buttons located in the orange area on the front panel and the blue rotary knob (navigation knob) on the right side are physically intact.
- The 2 oval buttons on the bottom printer unit are in place.
- The printer’s paper compartment lid is closed, and some thermal paper hangs out from the paper output slot, indicating that the mechanism is not jammed.
Electronic Components and Connection Points
The device is in the off position, thus no comment can be made from the visual regarding the screen’s pixel status or electronic functions. The connection sockets (SpO2, NIBP, FT) on the side panel appear clean and their pins undamaged. It can be observed externally that the pins are not bent. The black cable protruding from the side suggests that the device is connected to a power source or its cable is plugged in.
Accessories and Missing Items
The biggest deficiency in the visual is the absence of the intermediate cables (probes) required for measurement:
- SpO2 finger probe: Missing.
- NIBP cuff and hose: Missing.
- Temperature probe: Missing.
Only printer paper is present on the device.
Label Information and Symbols
The following technical inscriptions are readable on the device:
- UT3000A: Model code.
- SpO2: Blue-ringed input, for oxygen sensor.
- NIBP: Blood pressure measurement input (with patient type figures on it).
- FT: Temperature probe input.
- PRINTER: Bottom unit designation.
- Additionally, universal symbols such as menu, alarm mute, print, and paper feed are located on the keypads.
Battery Status
It cannot be determined from the visuals whether there is an internal battery, but it is highly probable that it has a battery since it is a portable device. No swelling (battery-related) is observed on the device casing.
Documents and Warranty
No documents such as an invoice, warranty certificate, or calibration label are present in the visuals.
Potential Risk of Malfunction
Although the general appearance of the device is robust, heavy dust accumulation suggests it may have been stored for a long time or remained unused. In such prolonged storage, there is a risk of internal batteries reaching the end of their lifespan. Furthermore, the dirt on the screen surface might be just dust or difficult-to-clean stains; however, there is no apparent risk of physical breakage. Due to the missing sensors, it should be considered that these consumables will incur additional costs when the device is acquired.