Ready For Sale
Secondhand Schröder Cura 3250 3 Motor Patient Bed
Price: USD$ 690,00 Approx: 31.050,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 16.02.2026
Schröder Cura 3250 3-Motor Patient Bed Analysis Report
Device Identification
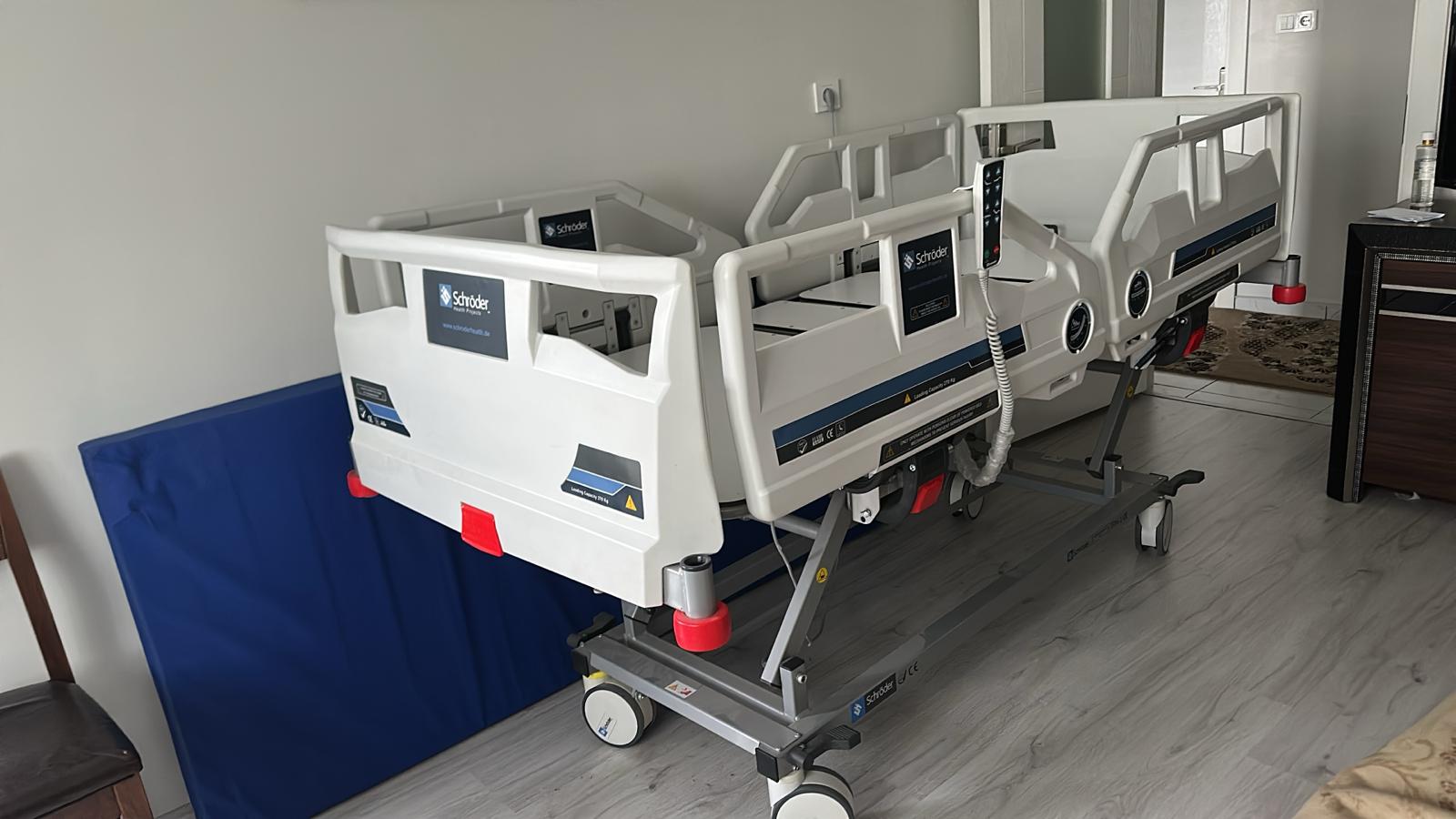
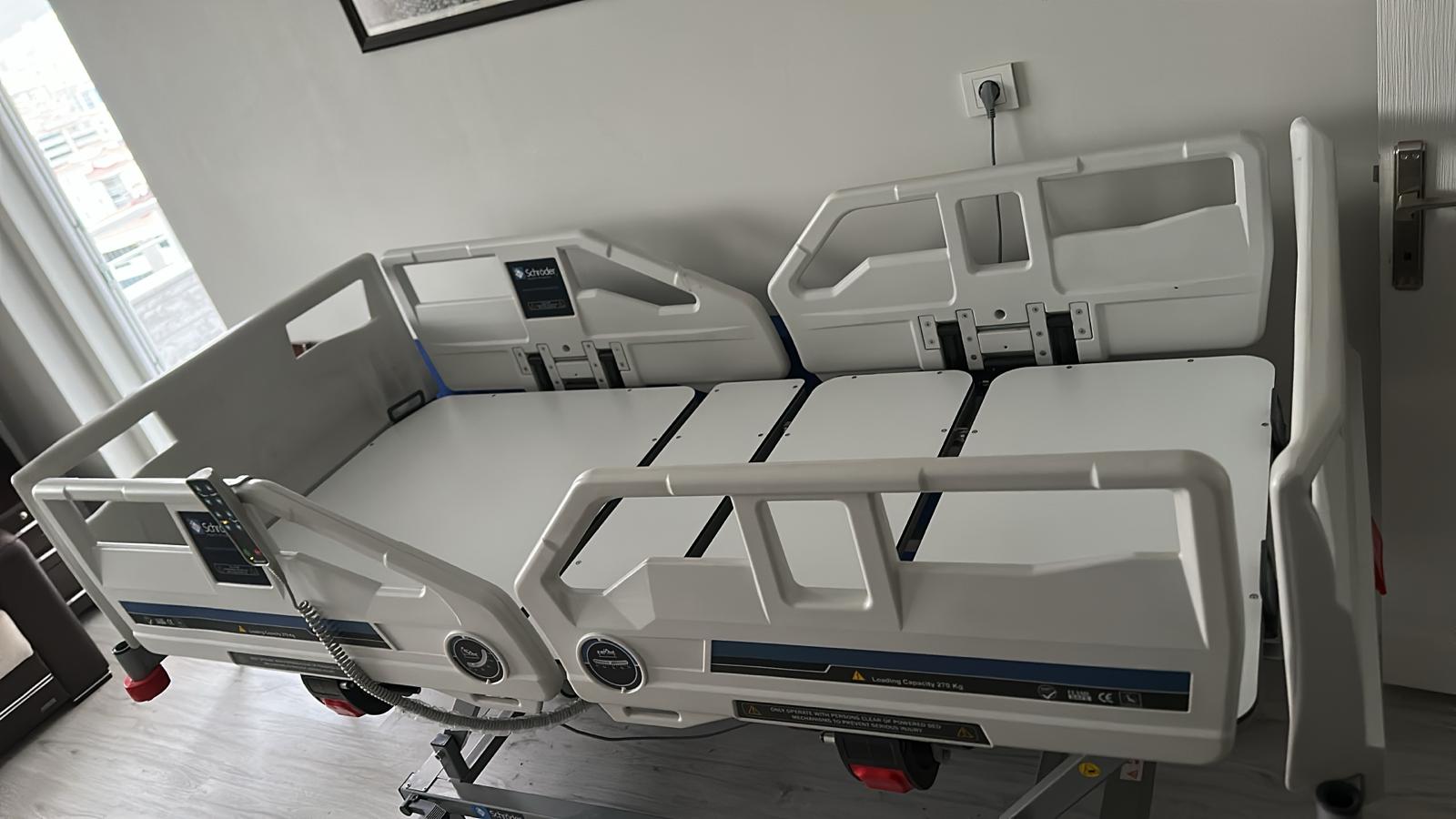
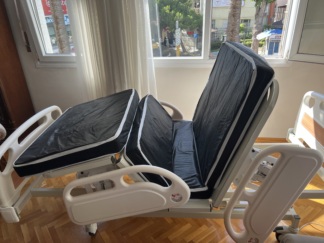
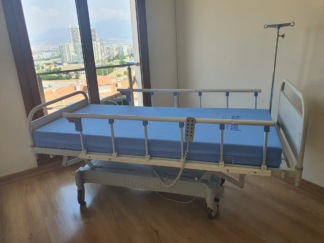
The product examined in the visuals is a motorized and electrically functional patient bed used for medical purposes, along with a compatible foam structure patient mattress. The device features a movable system capable of adjusting height, back, and foot sections to enhance patient comfort and ease care.
Brand and Model Information
Upon examining the labels and logos on the product, the following brand and model information has been definitively identified:
- Patient Bed Brand-Model: Schröder Cura 3250 (Stated as “3-Motor Patient Bed” on the label.)
- Patient Mattress Brand-Model: The mattress brand could not be identified as there is no visual of its brand or label in the images. However, a mattress compatible with the bed, with a blue protective cover, is present.
Originality
The “Schröder” and “Schröder Health Projects” logos, original product labels, CE marks, and “Made in Türkiye” inscriptions on the device indicate that the product is an original production of Schröder Medikal Tic. A.Ş. Traceable data such as barcodes, ÜTS number, and serial numbers on the label are the strongest evidence supporting originality. Plastic molds and workmanship quality conform to industrial standards.
Place of Manufacture and Origin
As clearly stated on the product label, the device is manufactured at the Gaziantep / Türkiye factory. The manufacturer is SCHRÖDER MEDİKAL TİC. A.Ş.
Areas of Use
This bed is designed for use in hospitals, nursing homes, and for patients requiring home care. The “Cura 3250” model is used in intensive care, general wards, or in situations requiring long-term home care; for adjusting the patient’s position (sitting, leg elevation, etc.), reducing the caregiver’s workload, and preventing patient falls (thanks to the side rails). Thanks to its CPR (Emergency Resuscitation) feature, it is suitable for use in emergency situations.
Quantity Information
The visuals show a total of 1 patient bed frame, 1 patient mattress with blue cover, and 1 wired hand control.
General Condition
The general condition of the device is visually nearly new and quite clean. No significant signs of wear were found on the metal parts, plastic side rails, or wheels. The label dates indicate that the device was manufactured very recently (early 2025), suggesting that it is almost in brand new condition.
Physical Deformation
No cracks, breaks, dents, or discoloration are observed on the white ABS plastic head and foot boards, side rails, or metal chassis of the bed. The plastic slats forming the mattress platform of the bed appear complete and intact. No paint peeling or rusting has been detected.
Mechanical Components
The device’s four-piece (butterfly-type) side rails appear sturdy. The smooth operation of the mechanisms is evident from the appearance of the side rails in both open and closed positions. The wheels and braking systems are visually in good condition. The red impact bumpers located at the corners are in place.
Electronic Components and Motor System
The label states that the product is “3-Motor”. From the visuals, the motor actuators and cable connections at the bottom of the bed can be distinguished. Additionally, control panels or angle indicators are visible on the outer parts of the side rails (in visual 5). The external appearance of the electronic components (cables, motors) is neat and undamaged.
Control and Accessories
The device includes a wired hand control. The control features the following functions:
- Back (Head) section up/down movement buttons.
- Foot section up/down movement buttons.
- Lift function (Entire bed up/down movement).
- Symbols for combined movement or Trendelenburg position.
- Green “Power” indicator and button.
- Red “CPR” (Quickly flatten bed in emergencies) button.
The control surface is clean, and there is no fading on the buttons.
Battery Status
A battery unit could not be directly identified from the visuals; however, in professional patient beds of this type, a backup battery system to counter power outages can often be integrated into the motor control box. Based on visual data, no definitive comment can be made regarding the presence or health of the battery.
Label Information
The technical label on the bed chassis (Visual 6) contains the following detailed information:
- Brand: Schröder
- Model: CURA 3250
- Description: 3-MOTOR PATIENT BED
- ÜTS Code: 8682139009762
- SN (Serial Number): 0701202561
- SPRNO: 03012025
- Load Capacity: The label on the side of the bed reads “Loading Capacity 275 Kg” (Safe Carrying Capacity 275 kg).
Year of Manufacture and Date Information
In light of the factory icon and date information on the label:
- Manufacturing Date: 07.01.2025 (Date next to the factory icon).
- Expiration / Warranty End / Validity Date: 07.01.2027 (Date next to the hourglass icon).
These dates indicate that approximately 1 year has passed since the device’s manufacture (Report date: 16.02.2026) and that the probable warranty or service period is ongoing.
Dimensions and Compatibility
It is of standard adult patient bed dimensions. The 275 kg load capacity warning indicates that the product has a durable, reinforced structure suitable for patients near the bariatric limit or heavy patients.
Documents
No physical paper invoice, user manual booklet, or warranty certificate is visible in the images. However, the label on the device contains a “Read User Manual” (book symbol) warning. The dated label serves as a reference for warranty tracking.
Potential Risk of Malfunction
There are no signs of corrosion, cable stripping, or physical damage on the device. Due to its recent manufacturing date, there is no observed risk regarding metal fatigue or motor lifespan. Only routine lubrication of moving parts and wheels, and periodic inspection of motor connections, might be required with regular use. Currently, no observed risk factors are present; the product is ready for use and in very good condition.