Ready For Sale
Secondhand Toshiba Capasee II Ultrasound Machine
Price: USD$ 960,00 Approx: 43.200,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 25.07.2025
Toshiba Capasee II Ultrasound Device Analysis Report
Overview and Device Identification
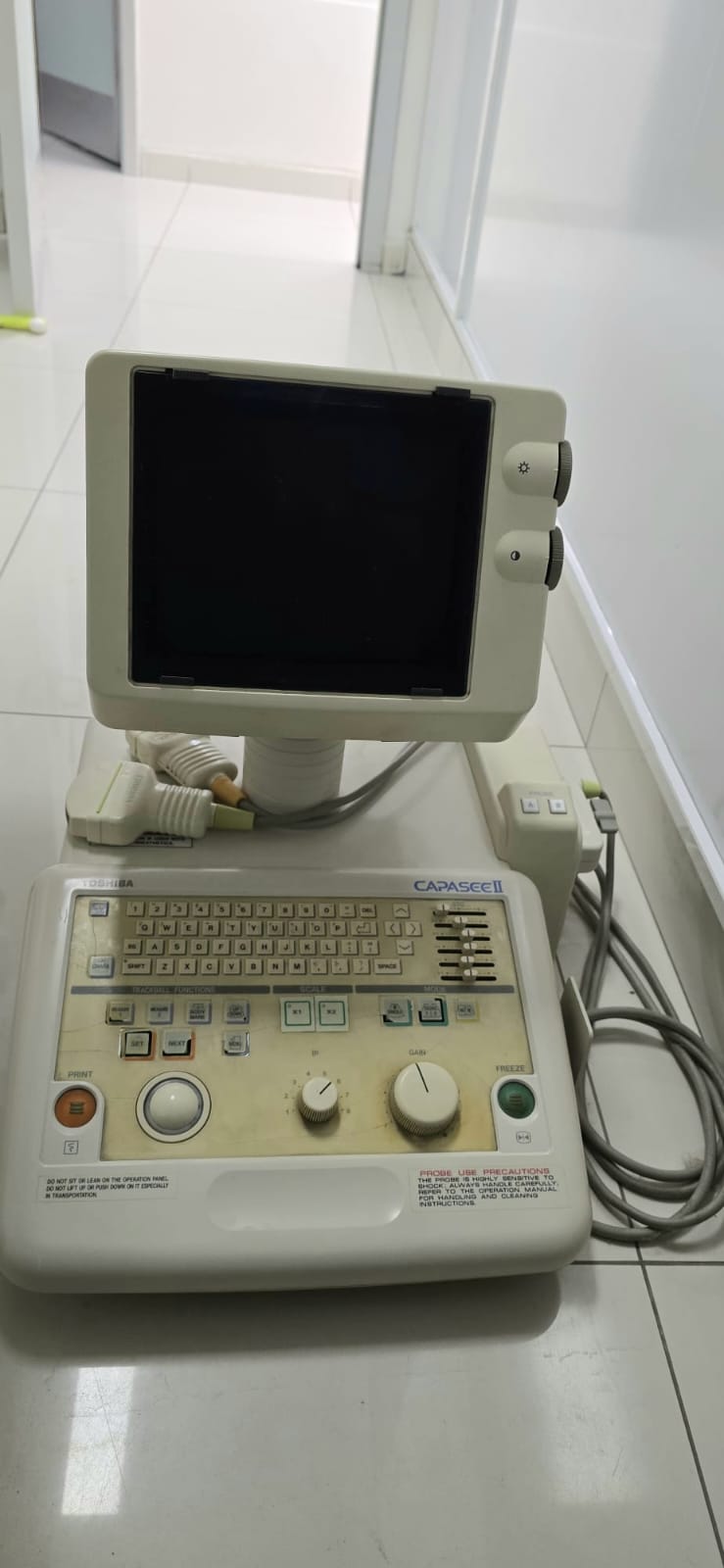
The product shown in the images is an ultrasound device used for medical diagnostic purposes. The device consists of a main console, a monitor integrated into this console, and ultrasound probes. The overall appearance of the product confirms that it is an imaging system used in medical environments.
This report is based solely on an analysis of the provided images and does not include a test of whether the device is operational.
- Identified Device: Ultrasound System
- Quantity Information: A total of 1 main unit (console and monitor), 2 ultrasound probes, and a power cable connected to the device have been identified in the images.
Brand and Model Information
The Toshiba brand and Capasee II model information are clearly written on the device’s control panel. Based on this information, the brand and model of the product have been definitively identified. The quality of the logo and lettering indicates that the product is an original Toshiba product.
Areas of Use
This device is a medical ultrasound system used for imaging organs and tissues within the body. The two probe inputs on the device indicate that various probes (e.g., abdominal, vascular) can be used for different diagnostic purposes. It is designed for various examinations in healthcare facilities such as clinics and hospitals.
General Status and Condition Assessment
The general condition of the device has been assessed as used. There is significant yellowing on its plastic casing, which has occurred over time and with use. Dirt accumulation has been observed, particularly around the buttons and trackball on the control panel. This indicates that the device has been in use for a long time.
Physical Deformation Analysis
Upon examining the physical condition of the product, the following details have been identified:
- Casing: The device’s white plastic casing shows general yellowing and discoloration. No cracks or major dents have been observed.
- Control Panel: The protective transparent membrane layer covering the control panel has cracks and peeling, especially on the upper left corner of the keyboard and over some function keys.
- Screen: The device’s screen is in the off state. No cracks or deep scratches are visible on the screen surface.
Mechanical and Electronic Components Examination
The visual inspection of mechanical and electronic components revealed the following findings:
- Mechanical Components: The buttons, adjustment wheels (knobs), and trackball on the control panel are physically in place. However, the heavy dirt buildup around the trackball and the large adjustment wheel indicates that the mechanical components have been subjected to heavy use.
- Electronic Components: There is no visual evidence regarding the functionality of the device’s electronic components, as the device is not powered on. The monitor is off, and there are no warnings or images on it.
Accessories and Connections
Two ultrasound probes are visible with the device in the images. These probes are plugged into the two sockets on the front panel of the device. No significant damage or crushing is visible on the probe cables. The power cable coming from the back of the device is also present in the image. No box, user manual, or other accessories are included in the images.
Labels and Warning Information
There are some warning labels on the device. However, the resolution of the images does not allow for the reading of detailed technical label information such as the serial number, REF code, or lot number. The readable warnings are as follows:
- PROBE USE PRECAUTIONS: There is a warning text indicating precautions for probe use, but its content is not legible.
- DO NOT SIT OR LEAN ON THE OPERATION PANEL: There is a warning label stating, “Do not sit or lean on the operation panel.”
Current and Potential Fault Assessment
Based on the visual inspections, the current and potential risks identified in the device are listed below.
Current Fault:
The cracks and peeling observed on the protective membrane covering the control panel are an existing physical defect. This condition could allow liquid or dust to seep under the keypad, potentially causing the keys to lose their functionality.
Potential Fault Risk:
The extensive yellowing of the device’s casing and the wear on the control panel indicate that the product has been in use for a long time. In such older electronic devices, there is a risk of future performance issues due to the lifespan of internal components. In particular, the growth of cracks on the control panel increases the risk of damage to the underlying electronic circuitry. These notes are only a forecast based on visual inspection and do not mean that a definite failure will occur.