Ready For Sale
Secondhand Erbe Erbokryo AE Cryotherapy Device
Price: USD$ 2.400,00 Approx: 108.000,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 12.02.2026
ERBE ERBOKRYO AE Cryosurgery Unit Analysis Report
Device Identification
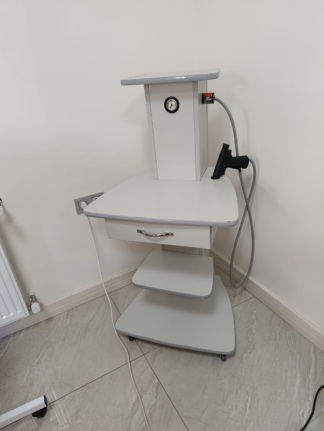
The main product examined in the visuals is a cryosurgery (freezing treatment) device mounted on a mobile transport stand (trolley) used for medical purposes. The system is a set consisting of a main control unit, a transport trolley, surgical procedure probes, and a foot pedal.
Brand and Model
The logo and model name located in the upper left corner of the product’s front panel are clearly legible. As a result of the examination:
- Brand: ERBE
- Model: ERBOKRYO AE
Areas of Use
ERBE ERBOKRYO AE is a device used in surgical procedures requiring tissue freezing (cryotherapy). Considering the structure of the probes in the visual and the typology of the device, it is suitable for tissue ablation or treatment in branches such as ophthalmology (eye), dermatology, gynecology, or general surgery.
Originality
The device’s front panel, logo prints, control buttons, and fonts used comply with industrial standards. The labeling on the product and the compatibility of the accessories indicate that the product is an original ERBE production.
Quantity Information
The number of parts in the set shown in the visuals is as follows:
- 1 Unit ERBE ERBOKRYO AE Main Unit
- 1 Unit Mobile Transport Trolley (Stand/Trolley)
- 1 Unit Black Foot Pedal (Foot Switch)
- 3 Units Packaged Probe/Applicator Cable
- 1 Unit Black Power/Adapter Cable (On the top shelf)
General Condition
The product is second-hand and used, in terms of overall condition. While the main device (console) appears clean, the transport stand shows noticeable signs of use.
Physical Deformation
- Device Casing: No significant dents or cracks are visible on the gray metal casing of the main unit. The front panel is clean.
- Transport Trolley: On the white metal parts of the transport trolley, especially on the rear vertical profiles and connection points, there are paint chips and associated signs of rust. Cosmetic wear and tear are noticeable on the metal parts.
Mechanical Components
The wheels of the transport trolley are in place. The glass of the pressure gauge (manometer) on the device’s front panel is intact. The control knobs (rotary black potentiometer and green on/off switch) appear physically sound.
Electronic Components
The device was not shown in working condition (not plugged in and screen lights not on), therefore, comments on its electronic functionality can only be made based on its physical appearance. The 2 digital display windows on the front panel are intact. No corrosion or breaks are visible in the socket inputs.
Accessories and Packaging Condition
The device’s most important accessories, the probes, are in sterile-looking packaging.
- Probes: 3 different cable/probe sets are located in pouches labeled “TYVEK” and “MEDSTER”. Date labels are present on the packages.
- Foot Pedal: 1 black, wired foot pedal, presumed to be compatible with the “ERBE” brand, is present. No crushing or breakage was detected in its cable in the visual.
- Power Adapter: An adapter/cable with a 3-pin input is visible on top of the device.
Label Information
The following dates can be read on the labels of the accessory packages:
- Expiration / Sterility Date: The date “29 04 2025” (April 29, 2025) is legible on the packages in the visuals. This information, when evaluated together with the “safecheck” notations on the packages, relates to the products’ shelf life or sterilization validity date.
- Yellow Label: Texts (AMCRY…) are written on a yellow tape manually affixed to the device’s front right corner; this is likely a code used by the previous institution for inventory tracking.
Display and Indicator Analysis
On the device’s front face:
- Left: Green “I/O” power switch.
- Middle: Two digital 7-segment display panels (one potentially for temperature, one for time/timer).
- Right: One analog manometer (pressure gauge). The needle is at the zero position, its glass is intact.
Dimensions and Compatibility
The product is desktop-sized but mounted on its original stand. It is suitable for standard operating room or polyclinic room dimensions. Accessory sockets are on the front face of the device, and the accompanying probes appear compatible with these sockets.
Existing Faults and Potential Risks
No significant breaks or missing parts (e.g., lost buttons, cracked screen) that would prevent the device from operating were detected in the visuals. However:
- Potential Risk: Paint chips on the transport stand may lead to metal fatigue or progression of rust due to ambient humidity. This situation affects the cosmetic and hygienic condition of the device rather than its electronic functionality.