Ready For Sale
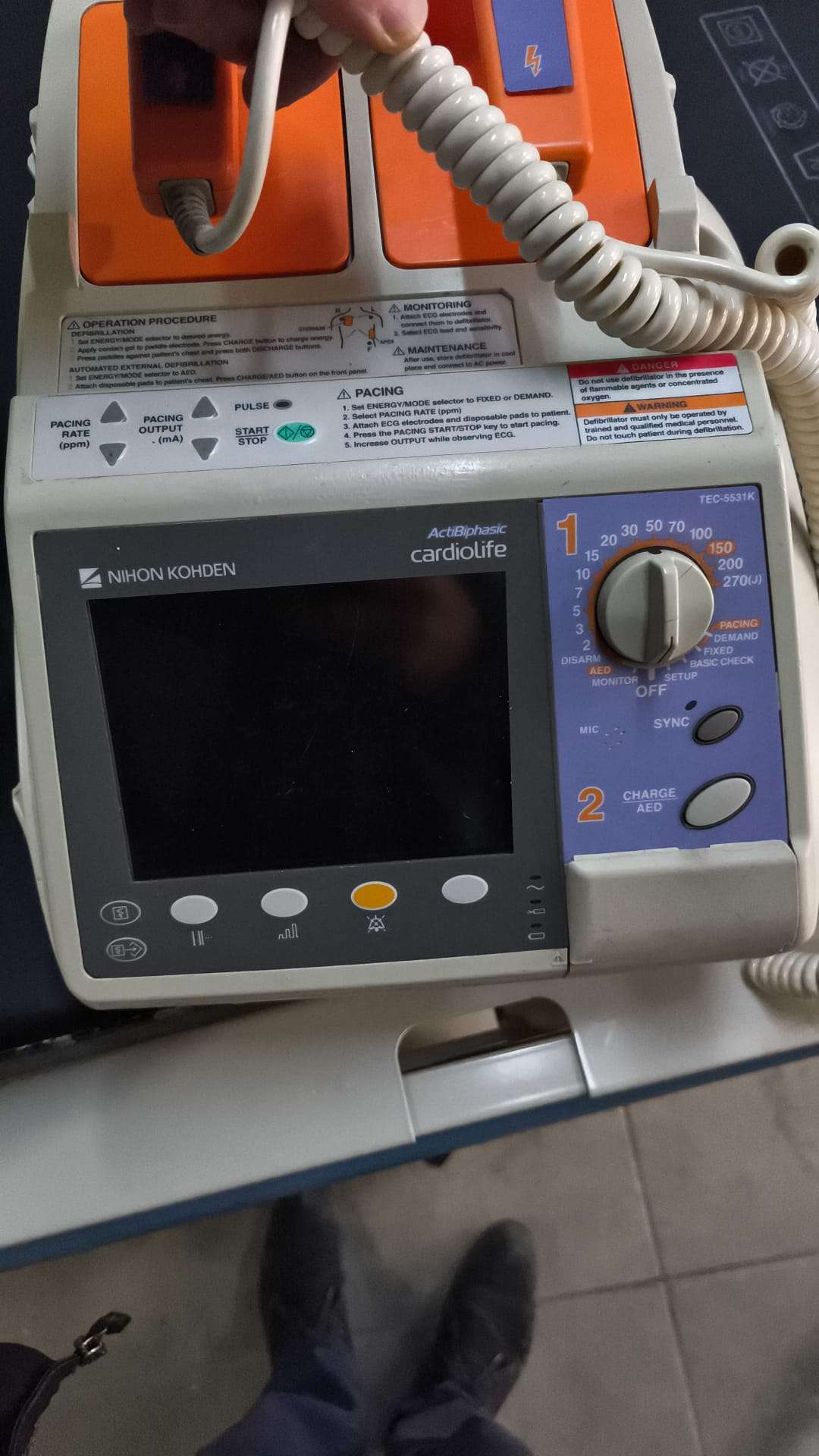
Secondhand Nihon Kohden Cardiolife TEC-5531K Monitor Defibrillator
Price: USD$ 1.640,00 Approx: 73.800,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 20.01.2026
NIHON KOHDEN Cardiolife TEC-5531K Defibrillator Analysis Report
Device Identification
The product in the images is a professional biphasic defibrillator device used to regulate heart rhythm or restart a stopped heart in medical emergencies. The device is also a monitor-featured unit with an external “pacing” (pacemaker) function.
Brand and Model
Upon detailed examination of the product’s front panel and its lettering, it is clearly understood from the model number in the upper right corner of the device that its brand is NIHON KOHDEN, its product family is Cardiolife, and its model is TEC-5531K. Furthermore, the panel indicates that the device features “ActiBiphasic” technology.
Originality
The brand logos, typographic print quality, color coding (orange paddles, grey-blue control panel), and industrial design features on the device indicate that the product is an original NIHON KOHDEN production. The alignment of the labels and material quality are consistent with original medical device standards.
Areas of Use
This device is designed for use in hospital emergency departments, intensive care units, ambulances, and operating theaters. Its primary uses include:
- Applying electrical shock (defibrillation) in life-threatening rhythm disorders such as ventricular fibrillation or pulseless ventricular tachycardia.
- Functioning as an external pacemaker in conditions like bradycardia, thanks to its “pacing” feature.
- Performing ECG monitoring as a patient monitor (when compatible cables are connected).
Quantity Information
The images show 1 unit of the main device and 1 pair (2 units) of external paddles with integrated wired connections to this device. It should be considered a single unit in total.
General Condition and Cosmetic Examination
The device generally maintains its integrity, but there are clear cosmetic signs of use. Yellowing, especially on the plastic components of the device’s outer casing (likely due to sunlight or time-dependent oxidation), is noticeable. Slight dirt accumulation due to use is present around the front panel buttons and on the handle areas. No major cracks or fractures are visible from the front.
Physical Deformation
No deep dents or fragmentation have been detected on the plastic frame of the front panel. However, it is observed that the grey casing of the device has lost its tonal uniformity in places. Especially around the edges of the paddle receptacles and on the handle grip areas, there are surface abrasions and slight color changes.
Mechanical Components
The rotary selection knob and the push buttons on the lower part of the device appear to be in place and intact. The paddles are seen to fit perfectly into their receptacles, and the locking mechanisms appear visually sound. No cracks are visible on the orange plastic bodies of the paddles.
Electronic Components
Since the device is powered off (de-energized), it is not possible to determine from the visual inspection whether the screen is functional or if there are pixel errors. However, no deep scratches or cracks are noticeable on the screen surface. No comments can be made regarding LED indicators and button illuminations as the device is off.
Accessories
The following are integrated into the device:
- External Paddles: A pair of adult-type shock paddles, mounted on the top of the device, with orange handles and marked “STERNUM” and “APEX”, are present. The shock and charge buttons on the paddles are visually intact.
- Spiral Cable: A white spiral cable connecting the paddles to the device is present. The cable retains its coiled form, but dirt accumulation is visible between the coils. No clear tears or peeling have been detected on the outer sheath of the cable from a visual perspective.
Note: Other accessories such as an ECG cable, power cable, or SpO2 sensor are not visible in the images.
Battery Status
Since the images only show the front and top parts of the device, no physical evidence can be provided regarding the presence or condition (swelling, leakage, etc.) of a battery, which is likely located at the back or bottom of the device. However, as it is a portable model, it should be noted that it has an internal battery compartment.
Label and Control Information
The labels and inscriptions on the front panel and top part of the device are legible:
- Energy Selection: Around the rotary knob, energy levels of 2, 3, 5, 7, 10, 15, 20, 30, 50, 70, 100, 150, 200, and 270 (J) Joules are marked.
- Pacing Mode: In the lower right section of the rotary knob, under the “PACING” heading, “DEMAND” and “FIXED” modes, along with “BASIC CHECK” and “SETUP” positions, are located.
- Warning Labels: On the upper part, there are English labels explaining operation procedures and containing high voltage warnings (Warning / Danger). There is no slight lifting or wear on the edges of the labels.
- Operation Sequence: Visual guidance numbers are present in the sequence: 1 (Green – Energy Selection), 2 (Orange – Charge), 3 (Red/Lightning – Shock).
Screen Analysis
The device features an LCD-type screen. The screen is currently off (black). As far as can be understood from reflections, no physical damage (internal breakage marks or surface cracks) is visible on the screen.
Year of Manufacture and Service Life
The “type label” showing the serial number and year of manufacture, likely located on the back of the device, is not visible in the images. Considering the device’s design and the degree of yellowing of its plastic components, it can be estimated that the device is not new and has been in use for a long time, but exact year data is not available.
Current Malfunctions
No obvious damage preventing the device from operating, such as broken parts, severed cables, or a shattered screen, has been detected during the visual inspection.
Potential Malfunction Risks
Internal breakages that may occur in spiral cables over the years cannot be understood from visual inspection, making them a potential risk point. Furthermore, as the yellowing of the device’s plastic components indicates its age, it is highly probable that the internal batteries (even if not visible) have reached the end of their life. The keypad and rotary knob, being the most frequently used mechanical parts, may carry a risk of sensitivity loss. However, they appear to be in good condition at present.