Ready For Sale
Secondhand Fisher-Paykel Airvo 2 High Flow Device
Price: USD$ 1.440,00 Approx: 63.360,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Free Shipping Conditions
You can benefit from our FREE SHIPPING campaign for your purchases in this category. Medbidding covers all shipping costs for both the buyer and the seller on orders that meet the conditions below. For all international sales and domestic sales within Turkiye that do not meet these conditions, Medbidding’s standard shipping procedures will apply.
Conditions
- The product must be sold for $350 USD or more.
- Both the buyer and the seller must be located in Turkiye.
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside of Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operations center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
- Technical Inspection: Technical inspections are performed by our business partner in the seller's country (where available) or by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 25.08.2025
Fisher & Paykel Airvo 2 Humidified Respiratory Device Analysis Report
Overview and Product Description
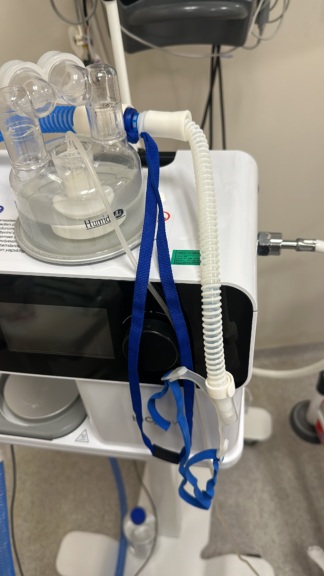
The product shown in the images is a Fisher & Paykel brand, Airvo 2 model humidification and high-flow respiratory support device. The product is used to provide respiratory support by delivering a heated and humidified air/oxygen mixture to spontaneously breathing patients. The device’s design indicates its suitability for a wide range of uses, from hospitals to long-term home care. The device, with its white and blue color scheme, features control buttons, a digital screen, and an air outlet port.
Package Contents and Quantity Information
According to the visual analysis, the products included in the package are listed below:
- 1 Fisher & Paykel Airvo 2 main unit
- 1 integrated power cable
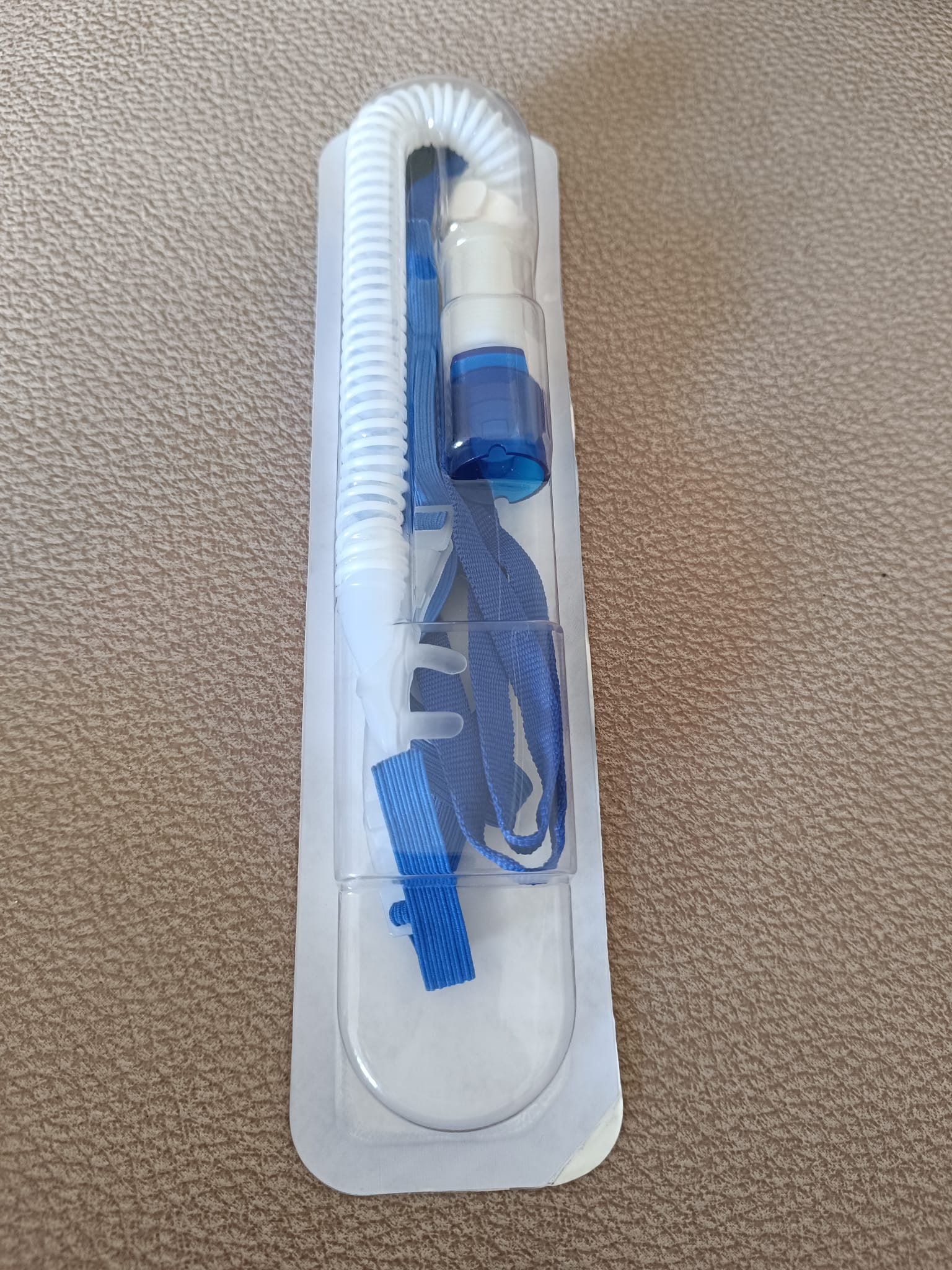
- 1 new, in-package breathing tube and cannula set
- 1 black carrying bag
- Various user manuals and information cards
Condition and State Assessment
The device generally appears to be in lightly used and good condition. No cracks, breaks, or significant discoloration were observed on the device’s plastic casing. There are slight circular usage marks on the metal heating plate located on the top. The control panel, buttons, and screen are physically clean and undamaged. There is a slight lift on one corner of the yellow warning label on the device, which reads “CHANGE ON schedule”. No crushing, breaks, or deformation were detected on the power cable and socket. The carrying bag is clean and its zippers are intact.
Technical Details and Label Information
The information on the device’s bottom label has been examined in detail. This information supports the device’s authenticity, and the technical details are as follows:
- Brand: Fisher & Paykel Healthcare
- Model: AIRVO 2 HUMIDIFIER
- Reference Code (REF): PT101EE
- Serial Number (SN): 200424124781
- Lot Number (LOT): 2101089508
- Manufacturing Date: 2020-04-24
- Place of Manufacture: New Zealand (Made in New Zealand)
- Protection Class: IP22
- CE Approval: CE 0123
Examination of Accessories
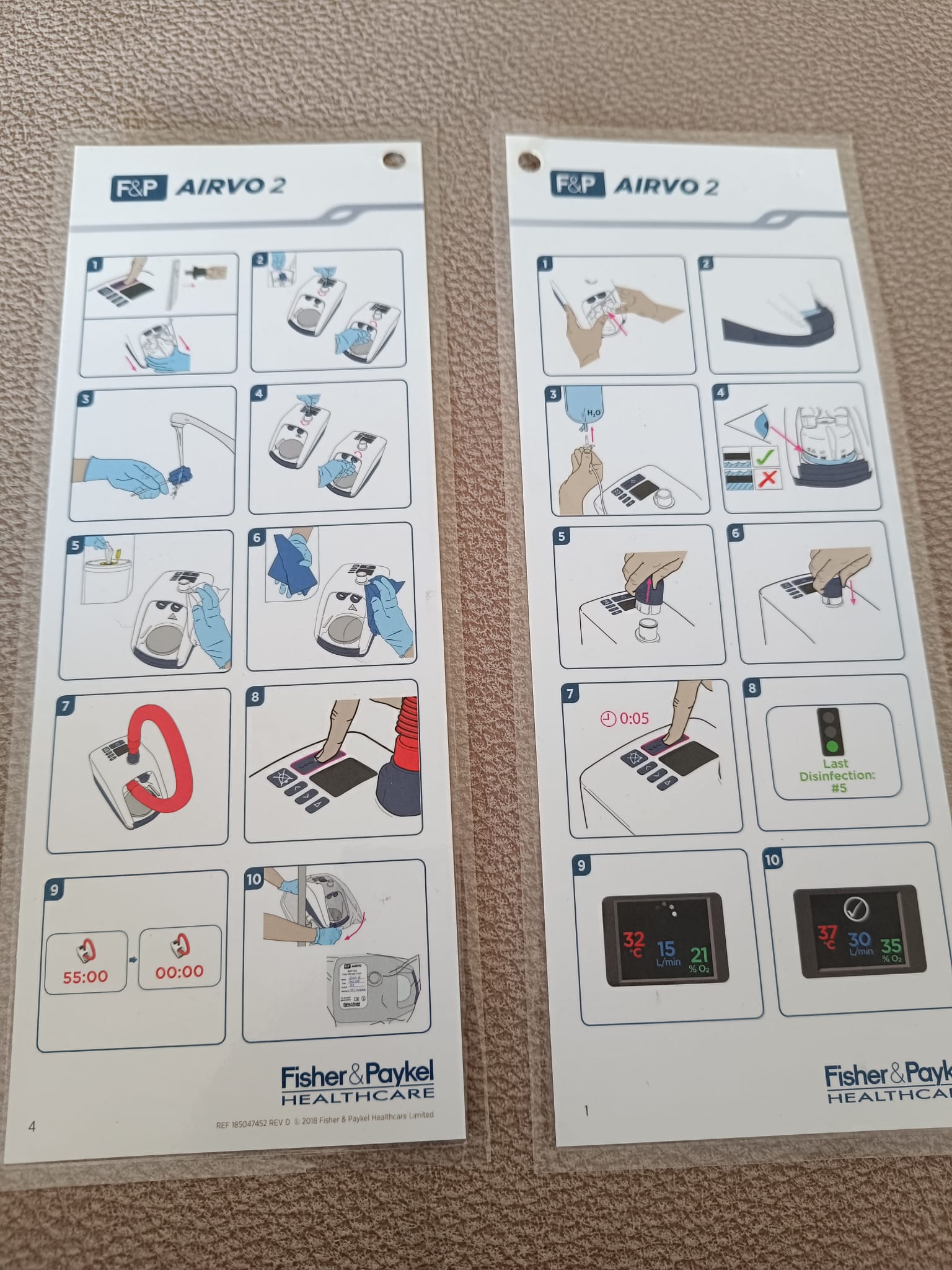
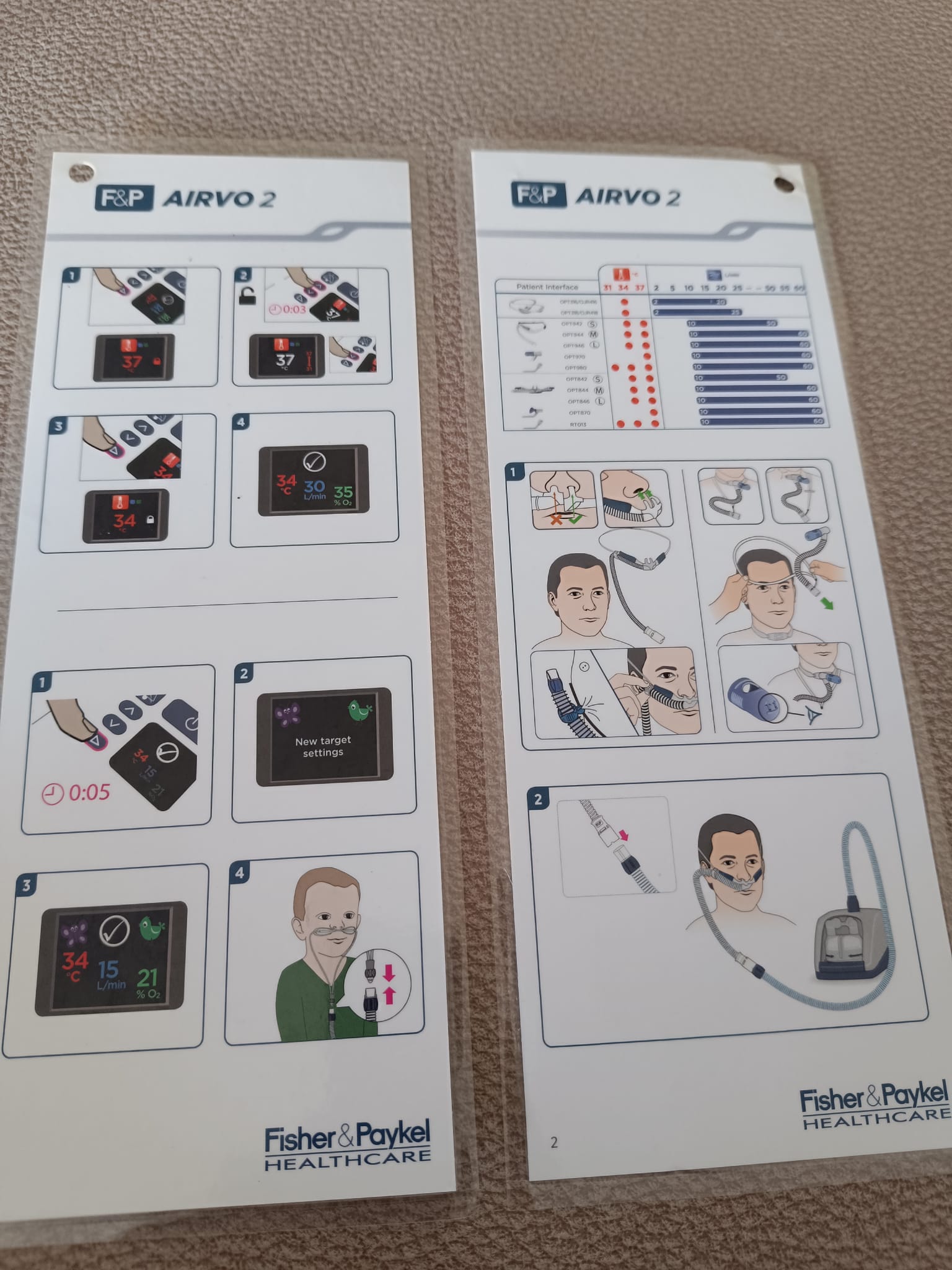
The accessories included with the device are a carrying bag, user manuals, and an unopened breathing set. The black carrying bag is designed to transport the device and its accessories and does not contain any tears or rips. The information cards in the images contain pictorial instructions on the device’s setup and usage. Information “REF 900247462 REV D © 2018” is readable on one card. The unopened breathing tube and cannula set is in a hygienic and unused condition. The expiration date could not be determined from the image.
Potential Risk Assessment
During the examination of the images, no serious physical damage, wear, rust, or crushed cables were found that could negatively affect the device’s operation or pose an immediate failure risk. The overall cleanliness of the device indicates that it has been well-maintained. The slight marks on the upper metal plate and the minor lift on the label are merely cosmetic imperfections and do not pose a risk to the device’s functionality. Based on its current condition, the device is expected to provide trouble-free use.