Ready For Sale
Secondhand Mindray DC-8 Ultrasound Machine
Price: USD$ 20.350,00 Approx: 915.750,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 23.01.2026
Mindray DC-8 Color Doppler Ultrasound System Analysis Report
Device Identification and Brand/Model Information
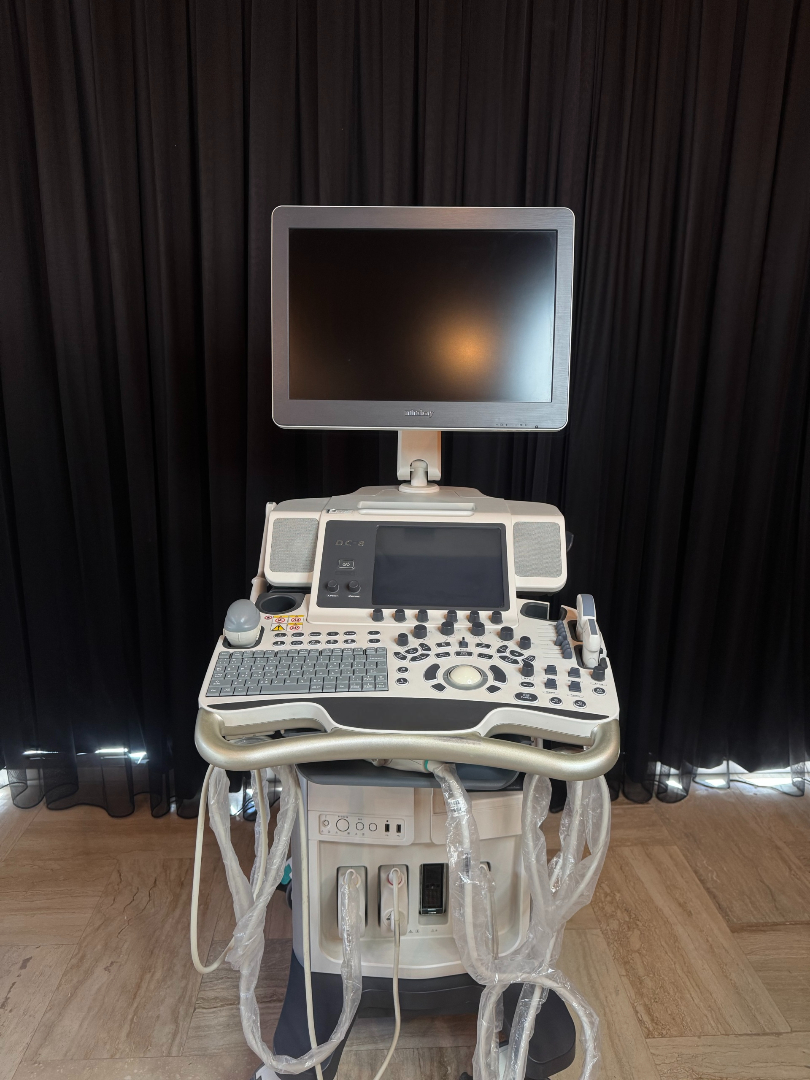
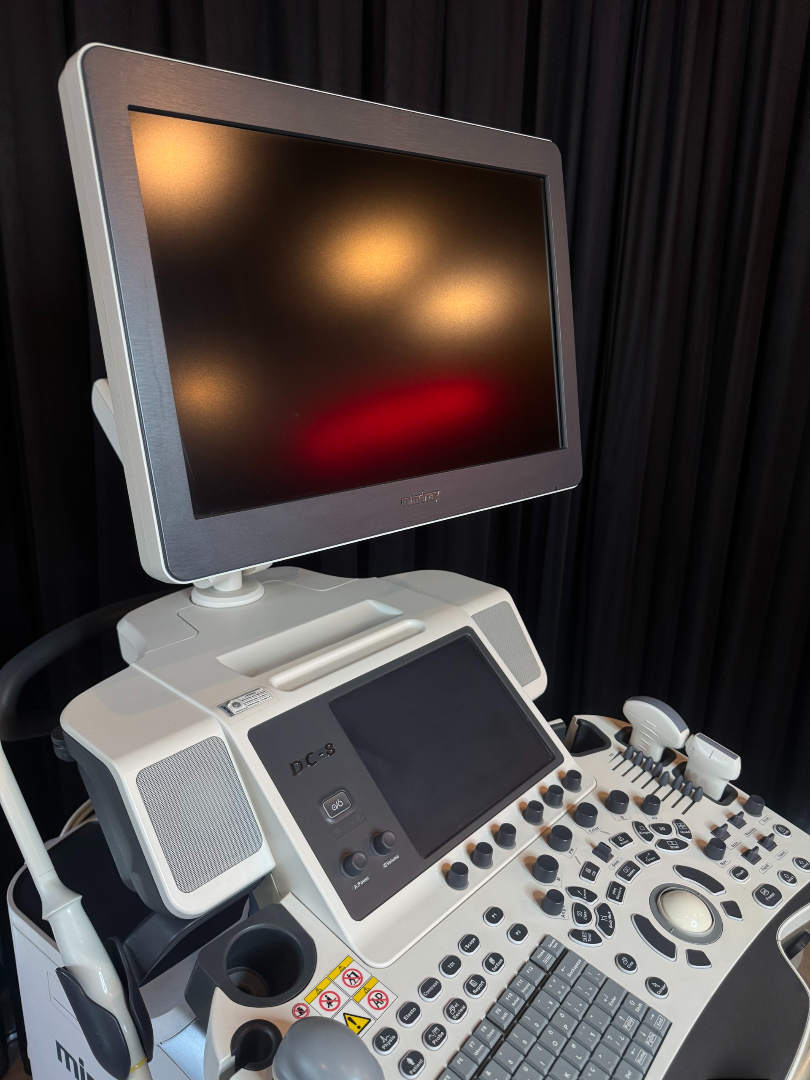
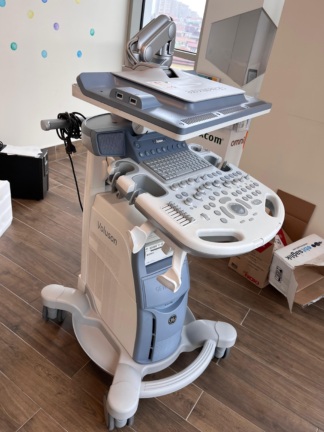
The device shown in the images is a console-type professional ultrasonography system used for medical imaging purposes. Upon examining the device’s design, control panel structure, the embossed logo behind the screen, and the labeling on the side body, the brand was definitively identified as Mindray. Upon inspecting the model indicators on the control panel and at the bottom left of the screen, the device’s model is clearly readable as DC-8.
Brand: Mindray
Model: DC-8
Considering the product’s workmanship, material quality, placement of brand logos (embossed logo behind the screen, print quality on side panels), and keyboard layout in the images, the device has been evaluated as an original Mindray product.
Areas of Use
The Mindray DC-8 is a high-performance medical imaging device used across a wide range of applications such as radiology, cardiology, obstetrics/gynecology (OB/GYN), urology, and vascular examinations. It is suitable for detailed internal organ imaging, pregnancy monitoring, and blood flow analysis (Doppler) in hospitals, imaging centers, and private clinics.
General Condition and Physical State
Upon examining the cosmetic condition of the device through the images, it appears to be very clean and well-maintained. No significant yellowing, deep scratches, breaks, or cracks were detected on the body plastic. The device retains its white and gray color tones, and despite being used, it gives the impression of being in “very clean” or “refurbished” condition.
- Physical Deformations: No noticeable dents, dings, or deformities are present on the outer casing, wheel base, or monitor frame.
- Mechanical Components: The articulated arm that supports and moves the main monitor appears sturdy. The mechanism for adjusting the height or angle of the control panel visually seems stable. No damage is observed on the carrying handles.
Technical Hardware and Screen Analysis
The device features two different display and control screens:
- Main Screen: A high-resolution LCD monitor is located at the top of the device. The monitor is in the off position, thus no comment can be made regarding pixel errors or image quality in an operational state. However, no deep scratches or impact marks are visible on the screen surface.
- Touch Command Screen: A smaller touchscreen for setting parameters is present immediately above the keypad. This screen is also off, but its physical integrity is complete.
Control Panel and Interface
The keyboard and control panel (UI) appear complete. The QWERTY alphanumeric keyboard, “Trackball”, TGC (Time Gain Compensation) adjustment knobs, and mode keys (Body Mark, Freeze, Print, etc.) are all in place. The writings on the keys are not erased, which suggests either infrequent use of the device or that the keypad has been renewed.
Accessories and Probe Connections
The images show 4 active probe input ports at the front bottom part of the device. Ultrasound probes are present on the device, but their cables and heads are carefully wrapped with transparent protective packaging materials.
Quantity Information: The images show 1 main device and at least 2 or 3 ultrasound probes/cables attached to the device within protective packaging (Due to packaging, the exact probe tip types and precise number cannot be clearly distinguished, but cable bundles are present).
Electronics and Potential Risk Analysis
Since no images of the device plugged in or powered on were shared, a visual analysis regarding the functionality of the electronic components, fan noise, battery status, or software version cannot be performed. However, based on visual cues, the following notes can be provided:
- Battery Status: The device’s internal battery is not visible from the outside, so its condition is uncertain.
- Risk Analysis: The careful wrapping of the device’s cables and probes suggests that the product is being prepared for shipment or has undergone a professional maintenance process. Visually, no rust, signs of liquid contact, or exposed hazardous cables are observed on the device. From this perspective, the device can be classified as visually safe and in good condition.
Label and Document Information
On the back of the device, below the “mindray” logo, there are electrical safety warning labels with a black exclamation mark on a yellow background. However, a detailed “Type Label” sticker containing the serial number, manufacturing date, or technical power values is not presented at a readable angle in the images. For this reason, the year of manufacture could not be determined.