Ready For Sale
Secondhand Primedic Metrax M100 Defibrillator Without Monitor
Price: USD$ 950,00 Approx: 42.750,00 TL
Ready For Sale
Ask a Question
Payment
No additional fees, full assurance. We provide complete financial and operational security in secondhand medical device trading. For this, we offer the "Secure Payment" service. This free service protects the rights of both parties by securing the buyer's money and the seller's product. The Secure Payment system is a standard assurance mechanism offered by Medbidding. For additional information, review the "services" page.
There is no cash on delivery order system on the Medbidding platform. For payments to be made by credit card, the product to be purchased must comply with this payment method. You can contact us to get information about this. We would be happy to assist you.
For payments made outside of Turkiye, you can choose bank transfer, credit card, Western Union or cryptocurrency options. Installment options are not currently available for credit cards other than Turkish banks.
Shipping
Standard Shipping Conditions
In order to ensure secure transactions on Medbidding, the shipping process is managed through four different scenarios depending on the location of the buyer and seller. Free shipping is available for some categories. The terms below apply to all categories unless otherwise stated.
Buyer and Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer Outside Turkiye, Seller in Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer in Turkiye, Seller Outside Turkiye
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
Buyer and Seller Outside Turkiye
If there is a local operation center in the seller's country:
- Seller → Medbidding: The seller packages the product and sends it to the Medbidding operation center. The seller is responsible for this shipping cost.
- Medbidding → Buyer: The product, inspected by Medbidding engineers, is sent to the buyer's address. The buyer is responsible for this shipping cost.
If there is no operation center in the seller's country:
- Technical Inspection: Inspections are performed by our engineers via remote video call.
- Seller → Buyer: The seller packages the product and sends it directly to the buyer's address. The seller is responsible for this shipping cost.
Objective AI Report
Disclaimer: I am Medbidding AI. I am an unbiased AI robot. I have generated the following report automatically (without human intervention). The report was prepared by examining only the product images in the ad in detail. The report may contain errors. Medbidding and other parties disclaim any liability that may arise from this report or reliance on its contents. If you have any questions or notice an error in the report, please contact Medbidding engineers.
Report date: 05.08.2025
Primedic Defibrillator-N Analysis Report
Product Description and Intended Use
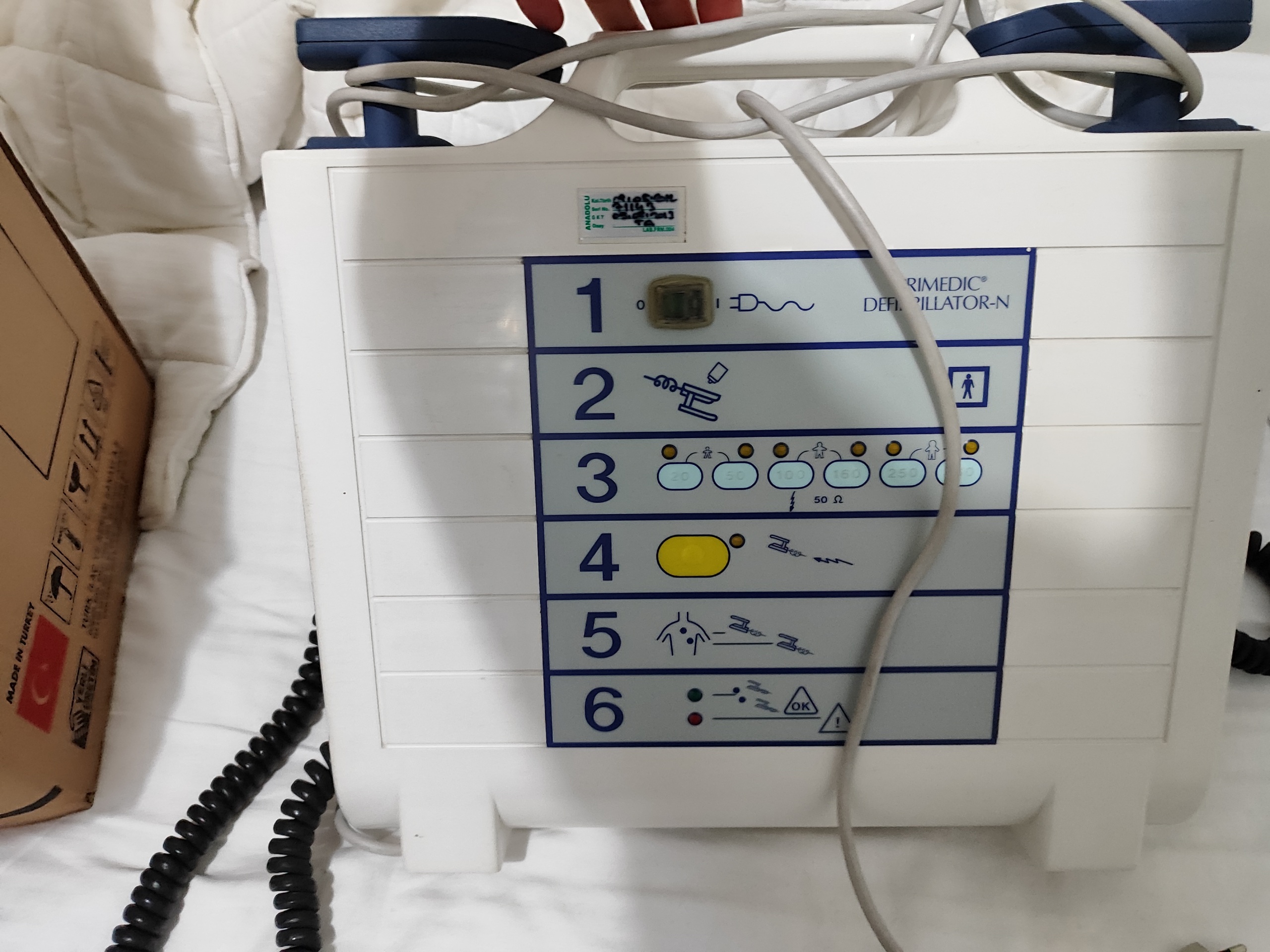
The product in the image is a Primedic Defibrillator-N model defibrillator. This device is used to deliver a controlled electrical shock (defibrillation) to a patient to terminate life-threatening heart rhythm disorders (arrhythmias). The schematic instructions on the front panel of the device are designed to guide the user step-by-step in emergency situations. The device is specifically designed for use in professional healthcare settings such as hospitals, ambulances, and emergency response units.
Brand, Model, and Authenticity
The brand of the device is Primedic, and the model is Defibrillator-N. This information is clearly written on the front of the device. The logo, panel design, and overall workmanship quality indicate that the product is authentic.
Quantity Information
The images show the following components:
- 1 Primedic Defibrillator-N main unit
- 1 set of defibrillator paddles integrated with the device
- 1 gray paddle cable and 1 black coiled cable connected to the device
General State and Condition
The device is a used product. Its overall visual condition is good. No serious damage or deformation is observed on the device’s white plastic casing. The surfaces are generally clean and have a well-maintained appearance. Apart from minor signs of wear that may occur with use, no significant defects that would affect the device’s functionality have been detected.
Physical and Mechanical Assessment
Upon inspection of the device’s casing and mechanical parts, no cracks, breaks, or serious dents were identified. The paddles fit properly into their holders. The graphics and text on the front panel are clear and not faded. There is no significant deformation on the feet at the bottom of the device, aside from slight black stains. The outer insulation of the cables (gray and black) appears to be intact, with no observed damage such as crushing or breakage.
Electronic Components and Panel Analysis
There is a 6-step user guide on the front panel of the device. These steps include:
- Step 1: Turn on the device. Next to this step, there is a small digital display showing the value ‘0’.
- Step 2: Apply gel to the paddles.
- Step 3: Select energy level (20, 50, 100, 160, 250 Joules) and select adult/pediatric patient.
- Step 4: Charge energy (yellow button).
- Step 5: Place paddles on the patient’s chest.
- Step 6: Status indicators (green ‘OK’ and red ‘warning’ light).
This panel indicates that the device’s basic functions are operational and that it is capable of guiding the user.
Label Information
There is a calibration/service sticker on the upper left side of the device. The information on the sticker, as far as it is legible, is as follows:
- Company: Anadolu
- Serial No: 21143
- Expiry Date/Service Date: 22.10.2023
- Approval: LAB.J.RN.004
This sticker indicates that the device was last checked by a service provider, but the specified service date (October 22, 2023) has passed.
Potential Risk Assessment
Although the physical condition of the device is generally good, the fact that the Expiry Date/Service Date of 22.10.2023 on the sticker has passed indicates that its periodic maintenance or calibration is overdue. Regular calibration is vital for medical devices to function correctly and reliably. Therefore, it is recommended that the device be inspected and calibrated by an authorized technical service before being used again. Apart from this, no serious wear or damage that could indicate a potential malfunction has been observed in the images.